Off-label, on meds
What does America's Food and Drug Administration know about the risks of transgender puberty blockers?

The gist
Judges have been asked to compel America’s Food and Drug Administration (FDA) to reveal what it knows about the off-label use of puberty blocker drugs by gender clinicians.
A Washington, D.C.-based conservative group, America First Legal (AFL), has launched a court action after the FDA failed to respond to a Freedom of Information Act (FOIA) request lodged last year.
“The recent whistleblower allegations about the Washington University Transgender Clinic [in Missouri] are just the latest evidence of dangerous experimentation on children with puberty blockers and cross-sex hormones,” AFL’s senior legal adviser Ian Prior said in a statement.
“While European nations are pulling back on these dangerous practices, the Biden Administration is moving ahead at warp speed.”
The FOIA request targeted eight FDA officials — in departments such as drug evaluation, endocrinology, paediatric medicine and drug labelling policy — as people likely to hold records on hormone suppression drugs including Lupron and Supprelin.
The drugs, known as gonadotropin-releasing hormone (GnRH) agonists or analogues, are approved by the FDA for a number of conditions including precocious (or unusually early) puberty, endometriosis and prostate cancer.
As is the case in other countries, these drugs do not have regulatory approval for blocking the unwanted sexual development of children diagnosed with gender dysphoria who identify as trans or non-binary. (Gender dysphoria or incongruence involves conflict between a person’s body and a felt “gender identity”.)
In a complaint filed last month in the U.S. District Court for the District of Columbia, America First Legal argued that the FDA had breached freedom of information law by failing to respond and release the records requested last September.
The AFL complaint asks the court to order the FDA to carry out immediate searches for the material.
Off-label prescriptions have a higher risk of “adverse drug events”, especially if this use lacks strong scientific evidence.
Dr David Gortler, a scholar at the Ethics and Public Policy Center in Washington, D.C. and a former senior adviser to the FDA commissioner for drug safety, told GCN that the use of a drug for an unapproved indication — in this case, gender dysphoria — “usually means that there is inadequate safety and efficacy data available.”
“If good safety and efficacy data exist, a sponsor [such as a pharmaceutical company] would be able to apply for an approved indication.”
Dr Gortler said AFL was “right to question something so obviously unscientific [as trans puberty blocking]” but could have better couched its arguments.
“Establishing drug safety and efficacy with the FDA are niche research areas that need specialised experts to frame questions when regulatory issues arise,” he said.
“In July 2022, the FDA issued a ‘black box warning’ for puberty blockers, the strictest kind of warning the FDA can give a medication. It issued the warning following evidence in patients of brain swelling and loss of vision. Despite this warning, doctors at the [Washington University Transgender Clinic in Missouri] continued their automatic practice of giving kids these drugs” — gender clinic whistleblower Jamie Reed, affidavit, 7 February 2023
Big news: The British Medical Journal calls out the low-evidence treatment guidelines driving the American surge in paediatric transition, while President Biden sticks to his catechism of gender change
The detail
Although often promoted as safe and fully reversible — a no-regrets pause in development — puberty blockers appear to lock-in gender dysphoria and medicalisation. On the limited data available, the vast majority of children started on blockers go on to cross-sex hormones, which are supposed to be taken lifelong.
If blockers are begun early and followed by these synthetic hormones, sterilisation is the expected result. For natal boys, early puberty blocking may render them incapable of orgasm. Other risks include low bone density, hot flashes and mood changes. The effects on the still-developing adolescent brain are unknown.
“At the moment [at age 27], I know that I would like to have children when I grow older, [while at the time I made the decision regarding starting treatment with puberty suppression [at age 17], I did not have a desire to have children] [...] That’s the only thing I wonder about, whether I was able enough to make that decision at the time” — interview with a natal female, journal article on decision making and puberty suppression, by psychologist Lieke Vrouenraets and Dutch colleagues, 17 September 2022
Identity medicine
Off-label prescription is not uncommon in paediatrics, sometimes because only the adult dosage for the same condition has been tested in clinical trials.
But in gender medicine, hormone suppression with gender dysphoria is different from its use with precocious puberty — the latter holds back development until the child can go through puberty in sync with peers — and the trans intervention takes place within a politicised context where clinicians and researchers have fundamental disagreements about diagnosis and treatment in the absence of a strong evidence base.
The evidence for the safety and efficacy of trans puberty blocking has been rated as weak and uncertain by systematic reviews of the medical literature. Patient numbers have skyrocketed, and the patient profile has changed without clear explanation.
The late 1990s, early 2000s Dutch innovation of puberty blockers for gender dysphoria has been adopted around the world, with children as young as 10 seeking a chemical stop for puberty, and there has been a parallel increase in concern about lack of long-term data, the risk of harm and the competence of children to give informed consent.
“Given paucity of evidence, the off-label use of drugs in gender dysphoria treatment largely means an unregulated live experiment on children” — Professor Carl Heneghan, director of the Centre of Evidence-based Medicine at the University of Oxford, The Times, 8 April 2019
Data points
Solid data on the extent of puberty blocking is hard to come by.
In researching her book on the collapse of the world’s largest youth gender clinic, the London-based Tavistock centre, journalist Hannah Barnes could not get a clear answer to the question of how many patients in total had been referred for blockers. It appears to be in the order of 1,000 over the ten years to 2021.
At Australia’s largest gender clinic, hosted by the Royal Children’s Hospital Melbourne, 59 patients started puberty blockers in the year ending 24 September 2022. On that date, the RCH clinic had 1,095 patients receiving unspecified treatment.
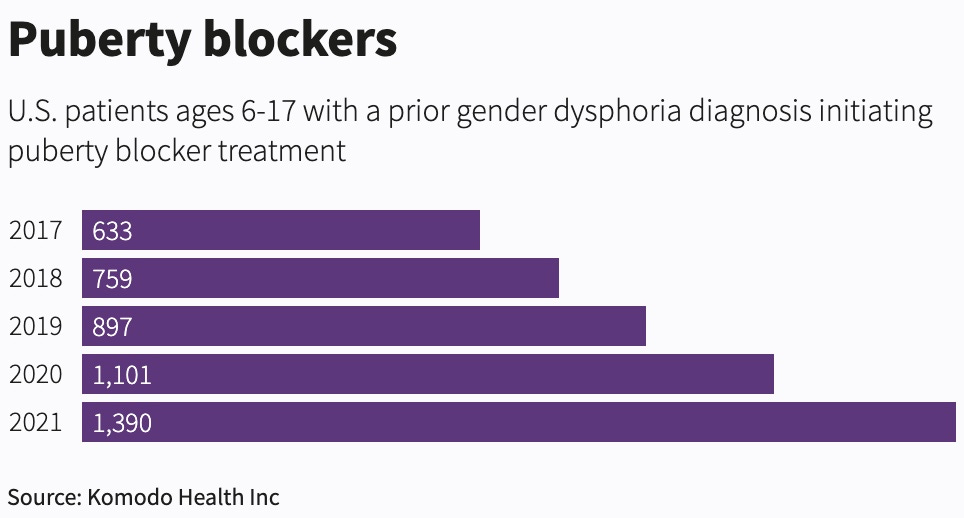
After an investigation in the U.S., the wire agency Reuters reported last October that, “Over the last five years, there were at least 4,780 adolescents who started on puberty blockers and had a prior gender dysphoria diagnosis.”
The U.S. data available to Reuters was limited, meaning its count is likely to be an underestimate. Data collection in gender medicine appears to be poor.
Risk-taking
In 2021, the FDA held a private “listening session with transgender adolescents to learn about their health care challenges and unmet medical needs,” the American Academy of Pediatrics reported.
“Adolescents’ perspectives on transgender health are important, as many transgender teens begin hormone therapies and hormone-blocking therapies around puberty,” the AAP said.
“[These minors aged 13-17] said the most important outcome is for their body (gender presentation) to match their internal identity. [They] described a willingness to accept risks associated with surgeries and treatments to achieve their goals related to gender transition and presentation.”
“It is my professional opinion that cross-sex hormones and puberty blockers should only be used where the benefits outweigh the harms. These drugs have imposed and are imposing serious harms on the children who have been patients at the [Missouri gender clinic]. The doctors at the [clinic] tell the public and tell parents of patients that puberty blockers are fully reversible. They really are not. They do lasting damage to the body. I have seen puberty blockers worsen the mental health outcomes of children. Children who have not contemplated suicide before being put on puberty blockers have attempted suicide after. Puberty blockers force children to go through premature menopause. Puberty blockers decrease bone density” — gender clinic whistleblower Jamie Reed, affidavit, 7 February 2023
#DetransAwareness
Adverse events
Asked if the FDA had a duty to address a dramatic increase in off-label use of puberty blockers, Dr Gortler said the roughly 35,000 adverse events reported to the FDA since the early 2000s “speak for themselves.”
“When considering those [adverse events], keep in mind the vast majority of those ~35,000 reports for that time period pre-dated the [gender medicine] advocacy of off-label use.
“[Yet] the FDA remains silent as the grave on the unstoppable biology-defying fundamentals of ‘blocking’ biological sex along with the clear drug safety problems detailed in [the FDA database of adverse events].”
Dr Gortler, a pharmacologist and molecular biology scientist who at one point worked on drug research for Pfizer, said he had included all adverse events with these hormone suppression drugs, regardless of the condition they were used for.
“I think it’s important to look at everything when considering an unapproved off-label indication, especially an off-label indication as unscientific and outrageous as trying to alter biological sex,” he told GCN.
“I included all generic names as well as chemical names and trade names used in the U.S. and around the world.
“The FDA has now begun speaking against the off-label use of medical devices, but remains conspicuously silent on puberty blockers and the administration of sex-incongruent hormones,” he said.
Buried in January’s 2023 omnibus appropriations bill was a new power for the FDA to restrict unsafe off-label uses of medical devices. There has been speculation this could lead to more FDA control over off-label drug use, although the agency is not supposed to interfere in the “practice of medicine.”
Last year, the FDA added a warning about the risk of pseudotumor cerebri — pressure within the brain that mimics symptoms of brain tumour — to the labelling of hormone suppression drugs for precocious puberty.
It cited six cases of this side effect in natal females aged 5 to 12. Five were being treated for the approved indication of precocious puberty, and one was for the off-label treatment of gender dysphoria.
“You can’t properly inform people about what is not known [regarding (possible consequences of) the treatment], except that there are uncertainties. That’s very difficult” — focus group of clinicians, journal article on decision making and puberty suppression, paper by psychologist Lieke Vrouenraets and Dutch colleagues, 17 September 2022
“We thought we were ‘buying time’ but we now realise that once a youth starts on blockers, they are likely to proceed to cross-sex hormones — that is not buying time but ‘colluding’, in my opinion” — Canadian child and adolescent psychiatrist Dr Susan Bradley, who founded the pioneering Toronto gender clinic in 1975, email to GCN, 14 March 2023.
“It doesn’t really mean much to a 12 year-old when you’re talking about osteoporosis [from low bone density]. She [my daughter] understood [what osteoporosis meant], but she thought ‘what does it matter, we’ll see about that later” — parent of a natal male, journal article on decision making and puberty suppression, by psychologist Lieke Vrouenraets and Dutch colleagues, 17 September 2022
“Despite the existence of published guidelines and the understanding that the effects of puberty-suppressing medication — the GnRH agonists — were temporary and reversible, no data were (or are) available on whether delaying the exposure of the brain to a sex steroid affects psychosexual, cognitive, emotional, or other neuropsychological maturation. Moreover, GnRH agonists for puberty suppression were neither governmentally approved nor publicly reimbursed; their use and paid supply within [Australia’s Westmead hospital] gender service was therefore ‘off-label’ and outside of an established legal or government-endorsed medical framework. These factors undermined clinicians’ felt sense of safety” — psychiatrist Dr Kasia Kozlowska and colleagues from the Children’s Hospital at Westmead, Sydney, journal article, 22 April 2021
Note: GCN sought comment from America First Legal. A spokeswoman for the FDA said it did not comment on “possible, pending or ongoing litigation”





It is interesting to compare this statement:
The evidence for the safety and efficacy of trans puberty blocking has been rated as weak and uncertain by systemic reviews of the medical literature. Patient numbers have skyrocketed, and the patient profile has changed without clear explanation.
With that of Melbourne’s Royal Children’s Hospital Gender Clinic website:
Puberty blockers suppress the development of secondary sex characteristics and are used for adolescents in the early stages of pubertal development. As they are reversible in their effects, should an adolescent wish to stop taking them at any time, their biological puberty will resume.
. . . . Nothing to see there!
Dynamite article, Bernard!