Gender on steroids
There's been a surge in young trans-identifying females embarking on lifelong testosterone drugs for a 'testicular disorder'
The gist
New data shows that more than 1,400 girls and young women in Australia started taking taxpayer-subsidised testosterone last year supposedly to treat the male disorder of androgen deficiency, which is typically caused by the testicles not doing their job.
This surprising trend follows a little-known rule change in 2015 removing the requirement that patients “must be male”, thereby enabling females who identify as the opposite sex or non-binary to get a public subsidy to masculinise their bodies with synthetic testosterone. The change was requested by an LGBTIQ+ lobby group, which complained of discrimination.
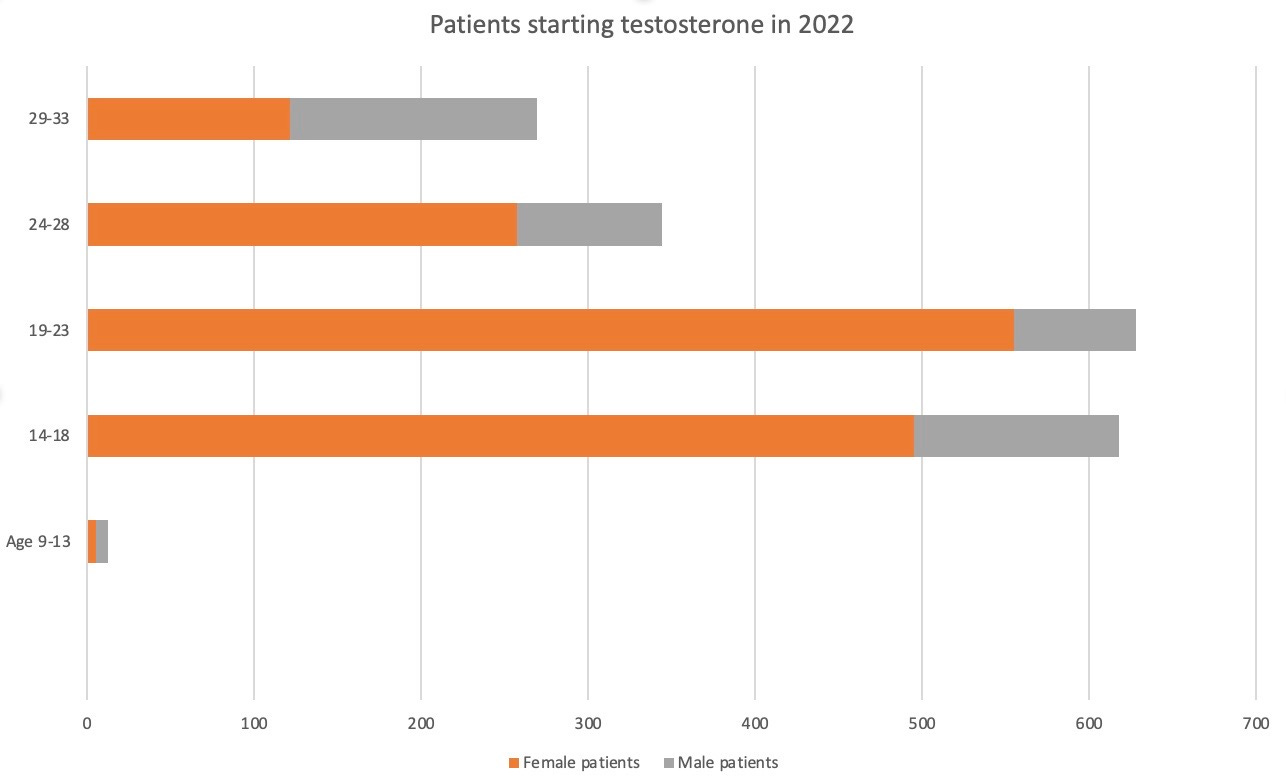
Last year, an estimated 1,433 females under the age of 34 started government-funded testosterone for androgen deficiency, almost 1,000 more than the number of males, who would indeed suffer from low levels of natural testosterone.
The female lead in new patient numbers subsidised by the Pharmaceutical Benefits Scheme (PBS) was most pronounced in the 14-28 age group, according to new data from the federal Department of Health and Aged Care. The figures do not include private prescriptions outside the PBS.
It is possible that the number of females starting testosterone is understated because for some years, trans-identifying biological females have been able to ask for their desired male “gender identity” to overwrite birth sex on their Medicare record, which flows through to PBS data, according to a spokesperson for the federal government agency Services Australia.
There is no PBS-funded testosterone listed to treat “gender dysphoria”—or gender incongruence, the latest “depathologised” term for a self-reported conflict between birth sex and a feeling of gender identity.
This use of testosterone is off-label, meaning the drug is not approved as a gender treatment by the Therapeutic Goods Administration, which would need to be presented with studies showing the efficacy and safety of testosterone for gender dysphoria.
However, this off-label use of testosterone “is a clinical decision made at the discretion of the prescriber who is responsible for obtaining informed consent from their patient”, a spokesperson for the Department of Health and Aged Care said.
There has been an unprecedented explosion internationally of sudden, atypical cases of gender dysphoria chiefly among teenage females, with one suggested cause being social influence online and through friendships groups.
In a 2019 Medical Journal of Australia (MJA) podcast, gender clinician and endocrinologist Dr Ada Cheung explained the prescribing rationale for biological females who identify as trans men. (Endocrinologists specialise in hormones.)
Dr Cheung acknowledged there was no PBS listing for testosterone to treat gender dysphoria, but said “transgender men are males, and they have a low testosterone level, so they have androgen deficiency, and they don’t have testicles.”
“So, we’ve been able to use the [PBS] indication ‘androgen deficiency due to an established testicular disorder.’ And when I’ve checked this with the PBS, they… have said that this is okay.”
Dr Cheung, who is a University of Melbourne researcher, was lead author of a “Position statement on the hormonal management of adult transgender and gender diverse individuals” published by the MJA in 2019.
That statement says, “For [female] people requiring masculinising hormone therapy for gender dysphoria, we use the authority indication ‘androgen deficiency due to an established testicular disorder’.”
“The diagnosis of androgen deficiency should be made only in men with consistent symptoms and signs, and unequivocally and repeatedly low serum testosterone levels… The initial diagnostic test in suspected androgen deficiency is measurement of fasting morning total testosterone in men...”—Chan et al, Androgen disorders update, Australian Family Physician, May 2014
A clinician’s domain?
GCN asked the Department of Health and Aged Care if the practice of prescribing PBS testosterone for females with the stated condition of “androgen deficiency” was compliant and legal.
A spokesperson did not give a direct answer, but noted that PBS testosterone requires a clinician to contact Services Australia for an authority to issue the prescription.
“Decisions about diagnosis, and selection of appropriate care and treatment for individual patients, [are] ultimately a matter for the prescriber in consultation with their patients,” the spokesperson said.
Chart: Young women and girls overtake males as new patients starting PBS-funded testosterone for androgen deficiency last year
Experimental treatment
Advocates for the radical “gender-affirming” treatment approach claim that opposite-sex hormone treatment (testosterone for females and oestrogen for males) improves mental health and “saves lives”; advocates campaign for easier access to these hormones funded by taxpayers and insurers, and often resist safeguards as “gatekeeping”.
Systematic reviews, which make it difficult for activist clinicians to cherry pick studies and misrepresent findings, have found the evidence base for hormonal treatment of minors with gender dysphoria to be very weak and uncertain, so much so that it has been described as “experimental” in Finland and Sweden. Systematic reviews in the United Kingdom declared the evidence to be of “very low” quality.
The risks of testosterone drugs for females include cardiovascular disease, vaginal atrophy leading to hysterectomy, sterilisation and sexual dysfunction. Girls who begin taking the hormone as young as 13 are expected to continue it lifelong, suggesting potentially more serious health risks than for the typical male patient aged 40 or older.
“Physical appearance can be irreversibly manipulated [with hormone drugs]; sex in its manifold and vital dimensions cannot be changed or eradicated. It is for this reason that persons manipulating their appearance and hormonal balance to achieve a cross-sex cosmetic effect remain lifelong clients of the medical-pharmaceutical industry. They must perpetually maintain the expensive and risky regimen of synthetic hormonal alteration of, and indeed resistance against, their [sexed] physiology, for the significance and composition of its genetic, cellular, and organic form never relents.”—American paediatric endocrinologist Dr Quentin Van Meter, expert testimony for a court case, 10 March 2020
Risks ignored
It was the lobby group now known as LGBTIQ+ Health Australia that successfully argued for the 2015 removal of the requirement that androgen deficient patients given PBS testosterone be male.
There was no discussion of the health risks for females exposed to testosterone in the “public summary document” from the relevant July 2015 meeting of the Pharmaceutical Benefits Advisory Committee (PBAC), the independent expert body which recommended that the health minister de-sex the PBS listing.
Unlike oestrogen drugs, testosterone is usually a tightly controlled substance because of its potential for abuse and health risks, as illustrated by the steroid scandal of East Germany in the 1980s and its aftermath of illness among former athletes. (Note: These athletes were given both anabolic steroids and testosterone, the steroids having different effects from the testosterone—GCN.)
“Would you like to help us understand pelvic pain and provide better care to trans and gender-diverse adolescents?’’—the Royal Children’s Hospital Melbourne gender clinic newsletter promotes research into pelvic pain commonly suffered by females on testosterone, early 2023
Minimal scrutiny
The 2015 PBS change allowing females to be given testosterone for androgen deficiency was approved as a “minor submission”, meaning there was no independent expert hearing, no analysis of the likely increases in use and cost, and no evidence from clinical trials. (Classifying the change as a minor submission reflected the nature of the request, according to the Department of Health and Aged Care.)
However, in a follow-up meeting in November 2015, the PBAC referred to “the complex nature of the submission”.
And the PBAC noted the argument by LGBTIQ+ Health Australia that the PBS listing for testosterone should not be changed to include the recognised diagnosis of gender dysphoria because the trans rights terms “gender affirmation and reparation” more correctly captured use of the drug.
For example, the PBAC reported the lobby group’s claim that some “non-binary patients”, who identify as neither male nor female, were denied treatment because it was not well understood that they used “testosterone intermittently, where they move in and out of treatment as necessary to achieve a preferred muscular aesthetic.”
The PBAC did not ask why a restricted drug such as testosterone should be made available for such non-medical reasons, according to the public summary of the meeting.
“The gender clinic prescribed me [as a young adult] with testosterone after just a 45-minute appointment. A few years later, I started experiencing serious abdominal pain. I found out that the years of testosterone had had devastating effects on my reproductive organs, so bad that [at age 24] I had a hysterectomy to remove my uterus, cervix, fallopian tubes and both ovaries, leaving me to grieve something that I will never understand.
“But I was still happy with my transition. I was uncomfortable with womanhood, and this was an escape. So I was happy with it for almost seven years, until I just wasn’t any more—until the regret crept in and pushed me so deep into a depression that I thought it would be impossible for me to make it out alive.”—American detransitioner Katie Anderson, who began social transition as a trans man at age 18.
Pharmacological sexism?
Apart from arguing that “lifelong testosterone administration is medically necessary for trans patients seeking this medication as part of medical gender affirmation”, LGBTIQ+ Health Australia claimed in 2015 that the restriction of PBS testosterone to males offended the federal Sex Discrimination Act.
In 2013, under Australia’s first female prime minister Julia Gillard, the definitions of a man and a woman were removed from that federal anti-discrimination law, and the novel idea of “gender identity” unmoored from birth sex was added as a characteristic to be shielded from discrimination.
Earlier this month, a new advocacy group led by gender-affirming family doctors discussed plans to apply for a PBS listing so that taxpayer-funded testosterone could be prescribed for gender incongruence—without the risk of clinicians relying on the claim that female patients suffer from androgen deficiency.
Video: ‘I watched a lot of gender-transformation videos and saw these people go from female to male, visually’
The detail
A 2020 report from the Drug Utilisation Sub-Committee of the PBAC, which had reviewed testosterone use, included in its key findings the fact that, “Females accounted for more first initiations of testosterone in 2018 than males in the 15-19, 20-24 and 25-29 year age groups.” Female usage had risen every year since 2015.
However, the sub-committee had nothing to say about the pros or cons of this development, apart from the fact that it “coincided” with the 2015 de-sexing of the PBS listing and an increasing number of new testosterone scripts written by practitioners of sexual health medicine.
This incuriosity seems odd given the startling nature of the change, not to mention a history of PBAC concern about the rising cost of PBS testosterone.
In an October 2015 budget estimates hearing, a senior official from the Department of Health and Aged Care, Adriana Platona, noted there had been “an exceedingly large usage of the [testosterone] product in the past five years.”
Ms Platona said PBS policy changes on testosterone had been “mostly aimed at limiting usage—and more appropriate usage—of those products in men over the age of 40.”
The concern was that family doctors (GPs) were writing scripts for a subset of men with androgen deficiency where the therapeutic effect of testosterone treatment was doubtful, imposing unnecessary health risks and public expense.
In 2011, PBS testosterone cost the taxpayer $14.6 million, up from $5.6m in 2005.
During the 2015 budget estimates hearing, Greens senator Janet Rice asked about the cost of puberty blocker drugs used off-label to suppress the normal puberty of patients she described as “transgender young people”.
Ms Platona’s response: “We need to encourage manufacturers of these [puberty blocker] products to apply for the PBS subsidy.”
“Up until recently we [in the health bureaucracy] have had no representation from the lesbian, gay, bisexual, transgender and intersex community. We have recently engaged with them in products that fall within their area of interest and will continue to work with them.”
Chart: Young women and older men on PBS testosterone

Closing the gate
In April 2015, against a background of concern about over-medication, the PBS listing of testosterone for androgen deficiency was changed so that GPs could no longer prescribe it without the involvement of a specialist.
But that looked like “gatekeeping” to the trans health lobby, which often frames access to hormonal and surgical interventions as “rights”.
At its July 2015 meeting, the PBAC considered two requests from LGBTIQ+ Health Australia—not only de-sexing the PBS listing, but also restoring the ability of GPs to prescribe testosterone without reference to a specialist.
LGBTIQ+ Health Australia said it was putting its case on behalf of “transgender and intersex patients”, although there is no evidence that the first group suffers from any hormonal disorders before they take synthetic opposite-sex hormones, while the second group is marked out as distinct by demonstrable medical conditions.
The lobby group asserted that “trans patients who are on a long-term testosterone therapy do not require the involvement of a specialist.”
The PBAC came down in favour of de-sexing the listing. It said making this change would be “relatively straightforward”. The public summary document from the meeting reveals no consideration of the efficacy or safety of testosterone treatment for females with gender dysphoria.
However, the PBAC did take into account a safety issue for males when it rejected the lobby group’s request for unchecked GP dispensing of testosterone, noting the “possible increased cardiovascular risk in older men.”
Chart: Male and female patients across all age groups commencing testosterone for androgen deficiency

A poor fit
The Australian Society of Plastic Surgeons recently applied for the creation of new item numbers under the Medicare Benefits Schedule (MBS) to subsidise the full range of trans surgeries including mastectomy and the creation of an artificial vagina.
The surgeons complained that existing item descriptors “are a ‘poor fit’ for performing gender-affirming [surgery] and cause anxiety to doctors using them as to whether they are using the appropriate MBS item.”
There are serious penalties for failing to comply with MBS or PBS rules.
In their submission, which shows the influence of gender-affirming jargon and advocacy, the plastic surgeons implied there was a similar anxious uncertainty in prescribing trans testosterone under the PBS.
They flagged a separate request by an unstated party for “a minor amendment” to the PBS listing. The proposal was to add gender incongruence to the clinical criteria for androgen deficiency, which would then read, “Patient must have an established pituitary or testicular disorder or gender incongruence.”
“Endocrine disorder, unspecified”—diagnosis stated on the new patient prescription for Helena Kerschner, who started testosterone at age 18. She went on to become one of America’s first well-known detransitioners. “Endocrine disorder” has been commonly used in the United States to bring treatment within insurance coverage; there is no evidence of trans-identifying adolescents suffering a hormonal imbalance before they start taking opposite-sex hormone drugs.
“Looking back in 2021, Ms Kerschner’s comment was: ‘diagnosed with endocrine disorder without even getting any tests done? How does that work?’ ”
Collaboration
A new doctors’ advocacy group—the Transgender and Gender-Diverse Healthcare special interest group of the Royal Australian College of General Practitioners (RACGP)—has been told of plans to apply for a PBS listing that would allow testosterone to be prescribed for gender incongruence. (General practitioners or GPs are doctors in primary care.)
At its November 13 meeting, the RACGP group heard that contact had been made with the pharmaceutical giant Bayer, one of the manufacturers of testosterone.
Two options were reportedly set out: Bayer itself could make the application and cover the fee for a PBS listing application; or Bayer could lend its application-writing expertise to others formally requesting the PBS change.
GCN acknowledges that gender-affirming clinicians genuinely believe their interventions help vulnerable youth. GCN sought comment from LGBTIQ+ Health Australia, Dr Cheung, Bayer, and the leadership of the RACGP special interest group.




Clearly the real issue is “cosmetic dysphoria” - as in “I don’t like the way I look”, which, if taken at face value (so to speak) would also include liposuction, face lifts, breast augmentation and so on. Why should a straight woman be denied publicly funded breast augmentation when a male can get it on demand and paid for I assume... why should any male be denied testosterone on demand in any quantity if they are self-identified as muscle dysphoric? A small quibble: the link on eastern German athletes was more about anabolic steroids, which can have fairly dramatic and painful impacts on liver health among other things, not on Teatosterone per se.
Until we pull the pernicious lie of "gender identity" out by the root clinicians will continue to promote the myth of "true trans."